
Key Takeaways
- Virtual clinical trials make participation easier by allowing patients to engage remotely, reducing travel and scheduling barriers.
- These trials improve accessibility and patient diversity, enabling people from different regions and backgrounds to take part.
- Digital tools like wearables, apps, and telehealth enable real-time data collection, improving monitoring and study accuracy.
- Virtual trials lower costs and speed up enrollment, making research more efficient for sponsors and participants alike.
Clinical research has gone through a noticeable change in recent years. Usually, long travel, tight schedules, and limited access make it hard for many people to take part in traditional studies.
As technology became a bigger part of daily life, a new approach started gaining momentum around 2019. It grew even faster during the COVID-19 pandemic, when researchers needed safer, more flexible ways to continue studies. This is where virtual clinical trials stepped in and changed how research could reach people across different locations.
In this blog, we will look at what these trials are, how they work, the benefits, the challenges, and what the future may look like.
What Is a Virtual Clinical Trial?
A virtual clinical trial is a study in which participants can complete most, or even all, trial activities remotely. Researchers use digital tools, telemedicine, mobile apps, and wearable devices to monitor participants and collect data in real time. Unlike traditional clinical trials, where patients must visit the research sites for assessments and follow-ups, virtual clinical trials allow participants to engage from home.
Although completely virtual trials exist, some studies use a hybrid approach where limited in-person visits are still required. This flexibility makes virtual trials more accessible and less disruptive to participants’ daily lives.
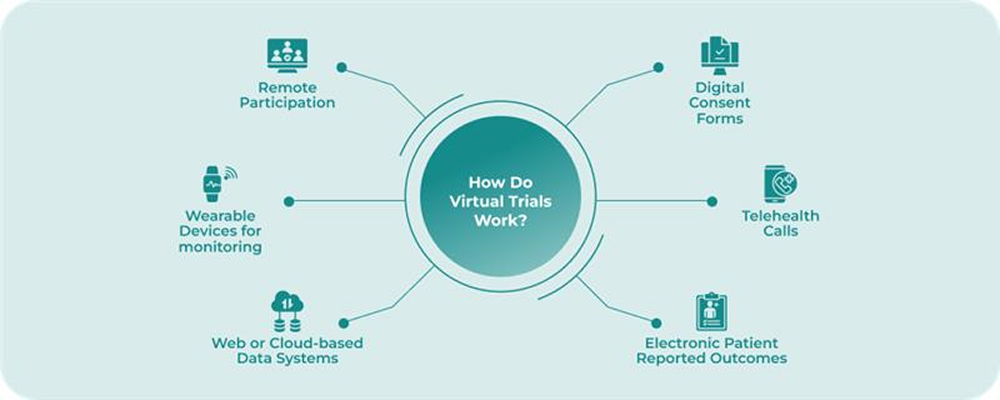
How Do Virtual Clinical Trials Work?
Virtual clinical trials use remote platforms to complete many steps of clinical research without requiring constant visits to physical sites.
You will see digital consent forms, telehealth calls, and wearable devices working together to collect data from the comfort of a participant’s home. In addition, cloud-based systems help store and share information in real-time so sponsors and research teams can stay updated throughout the study.
Furthermore, most of these trials also involve smartphone apps that guide participants through each requirement with reminders, educational material, and monitoring dashboards. Although some tests may still require physical checks, many tasks, such as surveys, symptom tracking, and medication logs happen online.
Key Benefits of Virtual Clinical Trials
Virtual clinical trials help both participants and sponsors by reducing pressure, improving communication, and enhancing data accuracy.
1: Increased Accessibility and Reduced Patient Burden
One of the key benefits of this approach is simple: fewer barriers. Many people cannot travel long distances or cannot adjust their schedules for frequent site visits. With virtual participation, travel is no longer required, timing becomes flexible, and tasks can be completed from home.
2: Improved Patient Diversity and Retention
Virtual systems help researchers reach people from different regions. Since participation is not limited to those living near major research institutes, more diverse groups can take part . When people feel supported, and the process feels comfortable, they tend to stay involved for longer periods.
3: Real-Time Data and Better Monitoring
Wearable devices and mobile health tools bring stronger data quality. These tools track information so that researchers can observe changes as they happen instead of relying on clinic visits. This approach makes the results more reliable and allows quick action if something needs attention.
4: Faster Enrollment and Lower Operational Costs
Virtual approaches make it easier to recruit more people in a shorter time. Since geography is no longer a barrier, enrollment grows faster. Also, sponsors save money on large facilities, travel reimbursements, and repeated on-site operations, which increases long-term efficiency.
Challenges with Virtual Clinical Trial
Virtual clinical trials make participation easier. They open new opportunities but also come with difficulties that need careful planning. Understanding the challenges of virtual clinical trials helps research teams prepare and respond better.
1: Technology Barriers and Digital Literacy
Some participants may not be familiar with using smartphones or digital tracking tools. Others may not have stable internet connections. These limitations can affect data quality or may slow down study progress.
2: Regulatory and Compliance Issues
Different countries have different rules for data privacy and medical monitoring. Getting approvals across regulatory bodies can take more time and may require extra documentation to ensure patient safety.
3: Data Security and Patient Privacy Concerns
Because these trials use digital platforms, researchers must protect the collected data. Weak security measures can increase risks and reduce participant trust. Therefore, the system must be strong enough to handle sensitive information without compromising patient details.
4: Difficulties in Remote Sample Collection or Procedures
Some studies require blood tests or specialized assessments that cannot be completed at home. Although mobile nurses or hybrid methods can help, they still add layers of coordination. These limitations highlight where virtual systems face obstacles.
Opportunities of Virtual Clinical Trials:
The opportunities for virtual clinical trials continue to grow. Wearable devices, mobile health apps, and telemedicine platforms are improving rapidly, allowing more accurate and continuous data collection. Patients are becoming more comfortable using remote healthcare technologies, which supports broader adoption of virtual trials.
Moreover, as healthcare systems adopt more digital tools, virtual trials can reach a wider range of people. This not only makes the results more representative but also helps advance medical research more efficiently. At the same time, it supports patient-centered research by making participation more convenient for everyone.
Virtual Clinical Trials vs Decentralized Clinical Trials
Decentralized Clinical Trials (DCTs) are a flexible type of research where some or all trial activities happen outside a regular clinic. This includes Virtual Clinical Trials (VCTs), which are fully done online or remotely. DCTs use technology and local support to make it easier for patients to participate and to collect data from real-life settings.
Future Outlook: How Virtual Clinical Trials Are Shaping Modern Research
Looking ahead, virtual clinical trials are expected to play a much bigger role in how global research evolves over the next few years. More sponsors are choosing remote and hybrid trial models because they support faster patient participation, smoother data collection, and stronger long-term efficiency.
Moreover, the market analysis suggests that the virtual clinical trials sector, valued at USD 9.77 billion in 2025, is projected to reach nearly USD 12.89 billion by 2030, representing a steady compound annual growth rate (CAGR) of 5.69 percent.
Overall, the future of clinical research is becoming more patient-centered and technology-driven. As these digital approaches continue to advance, virtual clinical trials are expected to help reduce trial delays, lower costs, and improve accessibility, establishing themselves as a vital part of modern medical research worldwide.
Conclusion
In summary, clinical research is evolving to be more patient-centered, accessible, and efficient. While challenges like technology adoption, regulatory requirements, and remote procedures remain, innovative solutions are helping overcome these hurdles and improve study outcomes. These advancements support faster enrollment, better monitoring, and broader participation, ultimately contributing to more reliable and inclusive results.
As clinical research continues to grow, Covalent Clinical Research is at the forefront, conducting patient-centered and inclusive studies in Mississippi. By prioritizing participant experience and high-quality study management, we are helping ensure research is both effective and meaningful. If you’re interested in being part of these studies, get in touch with us today to learn more and see how you can participate.

