
Key Takeaways
- 86% of trials struggle to meet recruitment timelines, highlighting the need for patient-centric clinical trials.
- Traditional designs often lead to slow enrollment and drop-outs, delaying drug development.
- Patient-centric approaches improve accessibility, communication, and overall participant experience.
- These practices lead to better recruitment, higher retention, and stronger data quality.
- Regulatory bodies now emphasize patient input as an essential part of modern trial design.
- The future of research is clear: trials must be built around patients, not protocols.
Patient-centric clinical trials are no longer optional; they are, transforming how research is designed and conducted. Sponsors, CROs, and research sites are putting patients’ needs, experiences, and safety at the heart of every study. These trials not only accelerate breakthroughs in drugs, medical devices, and digital therapeutics but also tackle one of the biggest challenges in research: recruitment.
With nearly 86% of clinical trials struggling to meet enrollment goals, adopting a patient-focused approach is essential to improve outcomes, build trust, and make participation more accessible. In this blog, we explore the core principles, benefits, and best practices of patient-centric clinical trials.
What is Patient Centricity in Clinical Trials?
Patient centricity in healthcare means putting patients first, whether that is making care more affordable or helping people better understand their health. In clinical research, it focuses on understanding what patients need, listening to their preferences, and improving their overall experience during a study.
Patient-centric trials are guided by three key principles: involvement, empathy, and transparency. These principles are most effective when clinical trials embrace diversity, ensuring that a wide range of patient perspectives is included and valued.
Applying these principles helps researchers create studies that genuinely listen to patients and align outcomes with what matters most to them. This means designing protocols, tools, and processes that consider patients’ convenience, comfort, and preferences at every stage. The FDA also supports patient-centered drug development, recognizing that incorporating patients’ voices leads to better-informed clinical decisions.
Regulatory Guidance on Patient-Centric Drug Development
Regulatory agencies and advisory groups are increasingly pushing clinical trial sponsors to adopt patient-centric approaches, supported by formal frameworks such as the FDA’s Patient-Focused Drug Development (PFDD) guidance.
Regulatory affairs guidance stresses that patients, as “experts in what it is like to live with their condition,” are uniquely equipped to shape the therapeutic context for drug development.
The message is clear: patient centricity in clinical trials is now an industry expectation, and its role in influencing trial quality, outcomes, and overall success will only continue to grow.
What Is the Patient Centric Approach in Clinical Trials?
The patient centric approach in clinical trials is a research model that prioritizes the needs, preferences, and lived experiences of participants at every stage of the study. Instead of designing trials solely around scientific or operational requirements, this approach ensures that patients’ perspectives shape protocol development, visit schedules, communication methods, and overall study design.
It aims to reduce participant burden, increase accessibility, and foster trust by incorporating patient feedback through advisory boards, surveys, and real-time engagement tools.
Ultimately, a patient-centric approach means conducting trials with informed consent. This also helps create trials that are easier to join, more comfortable to complete, and more reflective of real-world patient experiences, leading to better enrollment, higher retention, and more meaningful outcomes.
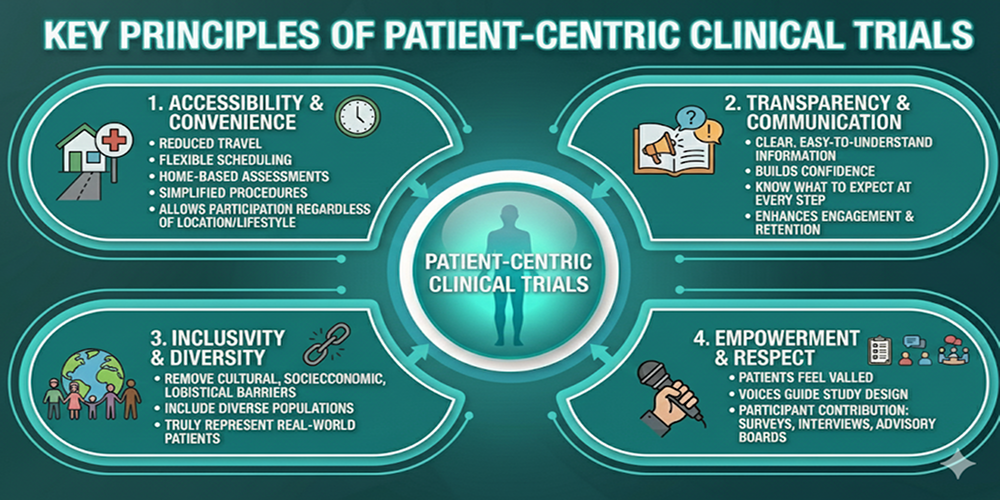
Key Principles of Patient-Centric Clinical Trials
To truly implement patient-centric clinical trials, organizations follow core principles that keep patient needs at the forefront:
1. Accessibility and Convenience
Reducing travel, offering flexible scheduling, supporting home-based assessments, and simplifying procedures allow more patients to participate regardless of their location or lifestyle.
2. Transparency and Communication
Clear, easy-to-understand information builds confidence. Patients should know what to expect at every step, which enhances engagement and retention.
3. Inclusivity and Diversity
A patient-focused design aims to remove cultural, and socioeconomic barriers so that trials include diverse populations that truly represent real-world patients.
4. Empowerment and Respect
Patients feel valued when their voices guide the study design. This is essential for patient-centered clinical trials, where participants contribute through surveys, interviews, and advisory boards.
Benefits of Patient-Centric Clinical Trials
There are many benefits of patient-centricity in clinical trials including:
1. Improved Enrollment Rates
When trials are built around patient needs, more individuals are willing and able to join studies.
2. Better Retention and Compliance
Simpler protocols, fewer site visits, and transparent communication reduce drop-out rates and improve data quality.
3. Higher Quality Data
Since patients are more engaged and supported, data becomes more reliable, consistent, and reflective of real-world behavior.
4. Enhanced Trust and Industry Reputation
Patients who feel respected and informed are more likely to remain engaged throughout the study and recommend participation to others.
Best Practices for Designing Patient-Centric Clinical Trials
To implement and scale patient-centric clinical trials, organizations should adopt proven best practices:
1. Involve Patients Early
Engage patients during protocol design to identify burdens, concerns, and opportunities for support.
2. Reduce Participant Burden
Use easy-to-understand language, make questionnaires shorter, avoid unnecessary tests, and keep visits to a minimum.
3. Provide Digital Support Tools
Apps, portals, and wearable devices allow patients to submit data easily and stay informed.
4. Prioritize Clear Communication
Use simple language and provide regular updates to ensure patients feel supported.
5. Offer Flexible Participation Options
Remote visits, mobile nursing, and hybrid models ensure inclusivity for patients with mobility, time, or distance barriers.
6. Train Study Teams on Patient Engagement
Staff should be skilled in empathetic communication and cultural sensitivity.
Why Patient-Centric Clinical Trials Are the Future
The shift toward patient-centric clinical trials marks a transformative moment in modern research. As trials become more personalized, compassionate, and accessible, patient experiences improve, and study outcomes become stronger.
Through decentralized trial models, smart use of technology, and clearer communication, patient-centric trials ensure that people, not processes, remain at the center of research. By adopting patient-focused approaches, sponsors and research teams can also speed up timelines, improve data quality, and build greater trust with participants.
Conclusion
Patient-centric clinical trials are more than an industry trend. They signal a fundamental reshaping of how research is designed, delivered, and experienced. While adopting patient-centric practices requires commitment, alignment, and continuous listening, the benefits are undeniable.
When patients feel understood and valued, trials become more inclusive, efficient, and impactful. Covalent Clinical Research ensures compliance and quality at every step, offering end-to-end services from patient recruitment and data management to regulatory guidance, helping your trials succeed.

