
Key Takeaways
- Clinical trials are medical research studies that test the new treatment to determine whether the investigational treatment is safe and effective.
- They progress through four key phases, each focusing on safety and results.
- The clinical trial process follows a structured plan called a protocol to ensure accuracy and participant safety.
- The main purpose of a clinical trial is not only to test the effectiveness of new treatments, but also to give patients access to innovative therapies.
Introduction
Every approved treatment you see in modern healthcare has once passed through a rigorous journey, whether it is common pain relievers or advanced cancer therapies. In fact, behind every approved drug, device, or treatment, there’s a long road of medical study, designed to answer one important question: Is this safe and effective for people? This is where clinical trials play a central role.
So, what are clinical trials, and why do they matter to patients and the medical community alike? In this blog, we will understand their purpose, the different types, and the step-by-step process behind them. You will also learn about their phases, the risks and benefits, and who is eligible to participate. By the end, you will have a complete understanding of why clinical trials are vital for advancing medicine.
What are Clinical Trials?
A clinical trial is a type of medical study that examines whether a new treatment is safe, effective, and superior to current options. Researchers test everything, such as new drugs, medical devices, diagnostic tools, and preventive measures.
Furthermore, the importance of clinical trials lies in the fact that they provide the only reliable method to test if a treatment truly works. So, when people ask, What are clinical trials? The answer is that it is more than just a test. It is a highly regulated process that ensures patient safety while collecting reliable data.
Moreover, before a trial begins, researchers spend years studying the treatment. It first goes through preclinical research to assess its safety and effectiveness, and after these safety checks, the investigational treatment is then evaluated in people.
Therefore, they play a vital role in advancing healthcare. They help answer questions like:
- Does the treatment work?
- Is it safe for long-term use?
- How does it compare to standard care?
Types of Clinical Trials
There are several types of clinical trials, each designed for different goals. Some test new treatments, while others focus on prevention, diagnosis, or quality of life.
Here are the main categories:
- Treatment Trials: Test new drugs, therapies, or combinations.
- Prevention Trials: Explore methods to lower the risk of disease.
- Diagnostic Trials: Develop better tests for early detection.
- Screening Trials: Identify diseases before symptoms appear.
- Quality-of-Life Trials: Improve comfort for patients with chronic illnesses.
After understanding the different types of clinical trials, it is important to understand how researchers ensure fairness and accuracy within these studies. Among the many study designs, one of the most trusted approaches is the randomized clinical trial.
So, what is a randomized clinical trial? And why is it the best way to study the safety and effectiveness of an investigational drug? Let’s explore this next.
Randomized Clinical Trials: A Study Design to Ensure Fair Results
When people ask, “What is a randomized clinical trial?” The simplest answer is that it is a type of medical study designed to test new treatments or interventions in a fair and unbiased way. Additionally, RCTs are often called the “gold standard” because they provide the most reliable answer to how effective treatment or intervention really is. After all, the participants are randomly assigned to different groups. This randomization removes bias and helps researchers compare results more accurately between those who receive investigational treatment and those who don’t.
In randomized clinical trials, participants are placed into different groups. One group receives the new treatment, while another gets either standard care or a placebo. Because no one can predict who goes into which group, the results are less likely to be influenced by outside factors or human bias.
Furthermore, randomization ensures that every participant has an equal chance of being placed in any group. This balance is what allows researchers to fairly compare outcomes. For example, if two groups start with similar characteristics, then differences in results are more likely due to the treatment itself rather than unrelated factors.
Randomized clinical trials help researchers:
- Eliminate bias by ensuring assignments are completely random.
- Strengthen accuracy since groups are more balanced.
- Improve reliability of results that guide future medical decisions.
Because of these advantages, randomized clinical trials are widely viewed as the gold standard in clinical research. They provide the most trustworthy evidence when determining whether a new therapy is safe and truly effective.
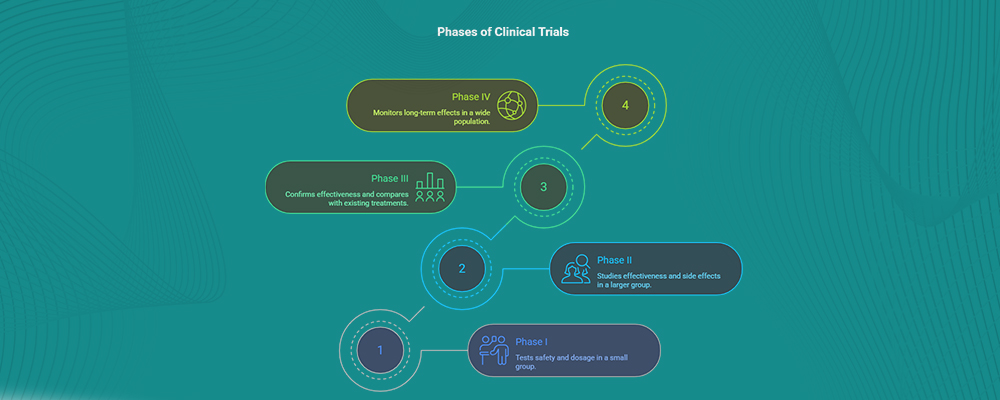
Phases of Clinical Trials Explained
To understand “what are clinical trials?” in detail, it’s essential to know the different phases of clinical trials, as each phase serves a unique purpose in testing the safety and effectiveness of a new treatment.
- Phase I: Tests safety and dosage in a small group of healthy volunteers or patients.
- Phase II: Studies effectiveness and side effects in a larger group.
- Phase III: Confirms effectiveness, monitors side effects, and compares with existing treatments.
- Phase IV: Conducted after approval, this phase monitors long-term effects in a wide population.
Therefore, each phase plays a critical role. For example, early trials may focus only on whether the treatment is safe. Later phases determine whether it works better than current options. Understanding these phases helps you see why clinical trials are both thorough and reliable.
The Clinical Trial Process
Now that we know the phases, let’s look at the clinical trial process. Each study follows a carefully designed plan known as a protocol.
Key steps of the clinical trial process include:
- Recruitment: Identifying eligible volunteers based on age, health, and other criteria.
- Informed Consent: Explaining the study in detail, including risks and benefits.
- Participation: Volunteers follow the treatment plan under close supervision.
- Data Collection: Researchers record results, side effects, and overall outcomes.
Throughout this process, safety is a top priority. Independent review boards and regulatory agencies monitor every step. This oversight ensures participants are protected while researchers gather meaningful data.
For patients, the process may seem complex, but it is designed with safety and clarity in mind.
What is the Main Purpose of a Clinical Trial
For patients enrolling in a clinical trial, one question they often ask is, “Why am I taking part in this study? What is the main purpose of a clinical trial?”
Here is the thing: The main clinical trial purpose is to test whether a new treatment, drug, or medical approach is safe and effective for people. But it’s also about hope, progress, and improving lives. Additionally, by participating in these trials, patients play an active role in helping doctors discover better treatment options for the future.
Moreover, clinical trials help determine if a new therapy can ease symptoms, work faster, or cause fewer side effects compared to existing treatments. Each study gives researchers valuable data, but it also allows patients to access innovative care that isn’t yet widely available.
Risks and Benefits of Clinical Trials
Clinical trials are designed to provide valuable insights into new treatments. Deciding to join a clinical trial is a personal choice. Like any medical decision, it comes with potential benefits and risks.
Possible Benefits:
- Access to cutting-edge treatments before they are widely available.
- Careful medical monitoring and support during the trial.
- Contribution to medical knowledge that could help countless others.
Possible Risks:
- New treatments may have side effects that are not fully known yet.
- The treatment might not work better than standard care.
- Participation may require extra time, travel, or tests.
Therefore, the important point is that every trial is closely monitored. Ethics boards and regulators are involved to ensure participants are protected.
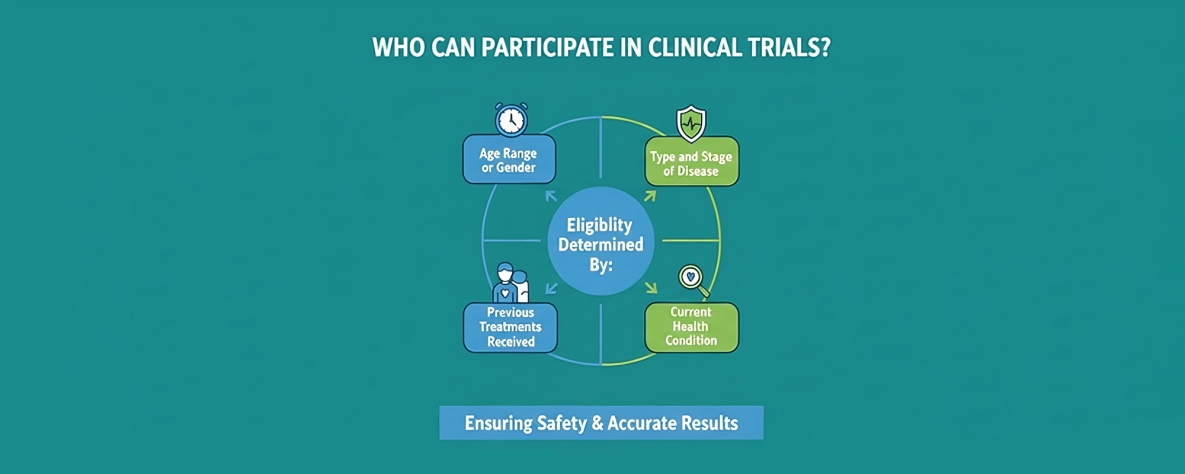
Who Can Participate in Clinical Trials?
Clinical trials follow specific guidelines to decide who is eligible to participate. These participation criteria are set by the research team and are part of the study’s official protocol. Furthermore, they help ensure that each study remains safe and produces accurate, meaningful results. These might include:
- Age range or gender
- Type and stage of disease
- Previous treatments received
- Current health condition
These criteria are in place to make sure the results are accurate and that participants remain safe.
Covalent Clinical Research: Advancing Medical Studies with Purpose
Understanding “what are clinical trials” is not just about knowing how studies work, but about recognizing the people and purpose behind them. Each trial represents a step toward finding safer, more effective treatments for patients who need them most.
At Covalent Clinical Research, we believe that research should always serve a greater purpose: improving lives. Additionally, we follow a patient-first approach, ensuring that every medical study is conducted with transparency, care, and strict adherence to ethical standards. From early-phase investigations to advanced trials, we focus on building trust with participants.
The Bottom Line
Understanding “what are clinical trials” helps us see how every breakthrough in medicine comes from years of careful research, patient participation, and scientific dedication. From randomized clinical trials to long-term follow-ups, each phase of clinical trials plays a vital role in bringing safer and more effective treatments to the world.
At Covalent Clinical Research, we’re committed to making that process clear, ethical, and accessible. We connect people with trusted research opportunities and guide them at every step. If you want to learn more about ongoing studies or explore participation, reach out to our team today. Your involvement could help advance the future of healthcare.

